
Cold fingers: A potential new salt transport process under lake ice
– By Jason Olsthoorn –
Introduction
The more we look under lake ice, the more unexpected things we find. Recent field campaigns have shown that photoplankton levels under lake ice can be almost as high as their peak summer values! Similarly, sunlight penetrating through ice and snow can cause thermal plumes that mix down to the bottom of even large lakes. What other unexpected processes are happening below the ice during winter?
The water under ice is usually density stratified. This stratification determines how much oxygen and other nutrients transport to the bottom of lakes, possibly controlling if the bottom water becomes hypoxic. The thermal stratification present before ice formation sets the stage for the rest of winter. In addition, salinity can also contribute to the density stratification. While most Canadian lakes are fresh ( S ≈ 0.2 g / kg), a small amount of salt can have a big effect on the stratification, especially in deep lakes.
If the salt stratification is large enough, it can prevent complete mixing of the lake each year.
During the winter, there are few processes that specifically transport salt. Sediment respiration, proposed by Mortimer and Mackereth (1958), would increase the salinity at the lake bottom, forming gravity currents that flow towards the deepest part of the lake. This is a bottom-up process. Are there other salt-transport processes and how important are they?
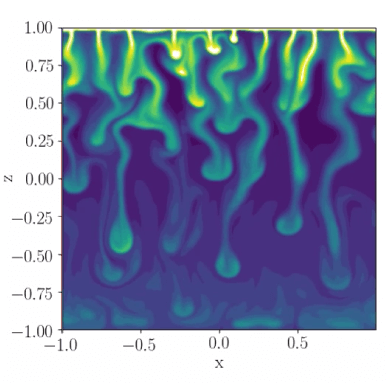
Our Model
When lake ice forms, it will reject contaminants, such as salts, into the water below. In collaboration with Dr. Cynthia Bluteau (U. Rimouski), Dr. Ted Tedford (UBC), and Dr. Greg Lawrence (UBC), we have shown that salt exclusion will lead to an increase in salinity under ice. This is a top-down process where salinity increases at the ice-water interface.
Both temperature and salinity affect the density under ice. The ice-rejected salt increases the fluid density, and, independent of anything else, would drive downward convection. However, temperature under the ice is stably stratified, meaning it can compensate for the extra mass of the salts. In this case, salt and temperature have opposing contributions to the fluid density. If there is enough salt in the water, and the lake-ice forms quickly enough, the water salinity will overwhelm the temperature stratification and the salt-exclusion will result in under-ice convection. However, most Canadian lakes are not very salty. What happens if temperature dominates the water density?
It turns out that heat and salt diffuse at different rates. These different diffusion rates can result
in what are known as Double Diffusive Instabilities. Using a simple numerical model and assuming a fixed salt flux from the ice, we showed that salt-exclusion forms double-diffusive plumes that are able to transport the saline water from the ice to the lake bottom, even if the sable temperature stratification controls the density. Our model predicted what these model plumes would look like and their effect on the vertical transport of heat.
To show that these saline plumes would happen in a real system, we performed laboratory experiments in a walk-in freezer, using a tank that was filled with 50cm× 50cm × 20cm of water. If the water we used was distilled, we did not observe any salt plumes forming under the growing surface ice. However, even for salinities as low as S = 0.02 g / kg, we were able to visualize salt fingers growing after a few hours. Because of the relatively small volume of water, the water did freeze faster than natural lake ice would grow, but controlling for the relevant parameters, we predict that we would find similar salt plumes in most Canadian lakes.

Does this happen in real lakes?
To our knowledge, no one has yet found salt plumes under lake ice. But this is not necessarily surprising given the complexities and sparseness of under-ice field measurements. In addition, these saline plumes transport salt, but they do not significantly mix the under-ice temperature stratification.
That said, we can estimate the mean ice growth for an average Boreal lake (50cm / Winter). Combining this with a global estimate for lake salinity and a guess at the under-ice temperature stratification, we estimate that most lakes are susceptible to these saline plumes for some portion of the year. This is particularly true at the start of the year, when ice grows quickest.
We can measure the salinity under lake ice, and we can show that it often increases over the winter. Is that a result of salt-exclusion, sediment respiration, or some other process that hasn’t yet been discovered? The verdict is still out, but I have a preference.
Relevant Papers
Olsthoorn, J., Bluteau, C.E. and Lawrence, G.A. (2020), Under-ice salinity transport in low-salinity waterbodies. Limnol Oceanogr, 65: 247-259. https://doi.org/10.1002/lno.11295
Olsthoorn, J., Tedford, E.W.. and Lawrence, G.A. Salt-fingering in seasonally ice-covered lakes. GRL. Under Review.
Dr. Jason Olsthoorn is an Assistant Professor at Queen’s University, where he models mixing and transport processes that occur in Canadian lakes. He recently completed his Killam Postdoctoral Fellowship at UBC, after completing his PhD at the University of Cambridge. He loves to cook, which may explain his fascination with salt.
