
Yes, there is selenium in our atmosphere: a quick look into recent discoveries about its biogeochemical cycle.
– By Paul Heine –
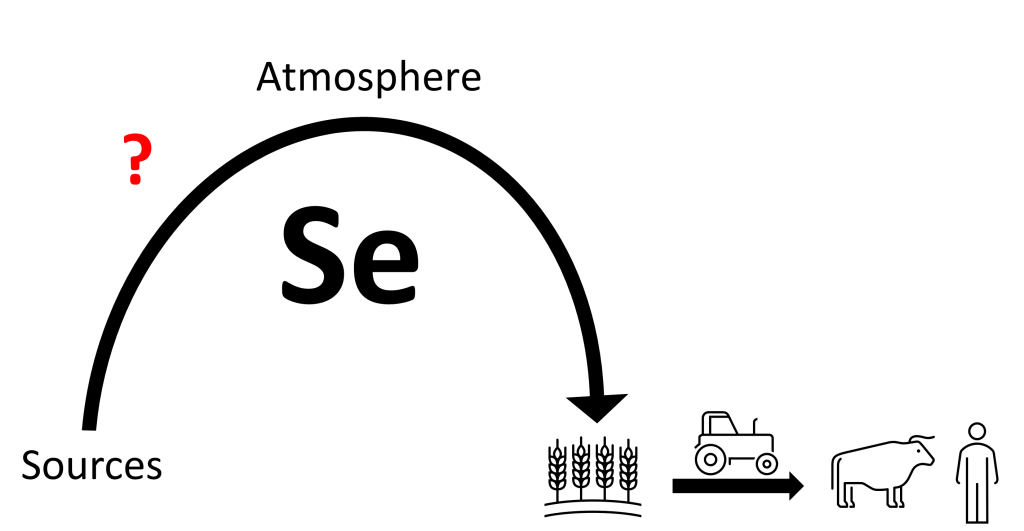
Selenium (Se) is an element with similar chemical properties compared to elements like sulfur. Just like sulfur, Se can be found naturally in bedrock, soils, the atmosphere, and marine systems, but also in plants. As part of their metabolism, plants take up Se from soils, to incorporate into amino acids and build proteins. These Se containing proteins serve vital functions for both animals and humans, thus making Se an essential micronutrient. Contrarily, exposure to slightly higher amounts of Se can result in adverse health effects or even death. Therefore, it is important to ensure that food for both animals and humans contains just the right amount of Se. Unfortunately, there are estimations that up to 1 billion people globally do not have an adequate amount of Se in their diet. The dominant cause of this is low levels of Se in soils, which limits plants’ ability to metabolize Se and consequently leads to deficient levels of Se in food stuff. But how exactly does Se get transported through the environment to soils to ultimately end up in people’s food (Figure 1)?
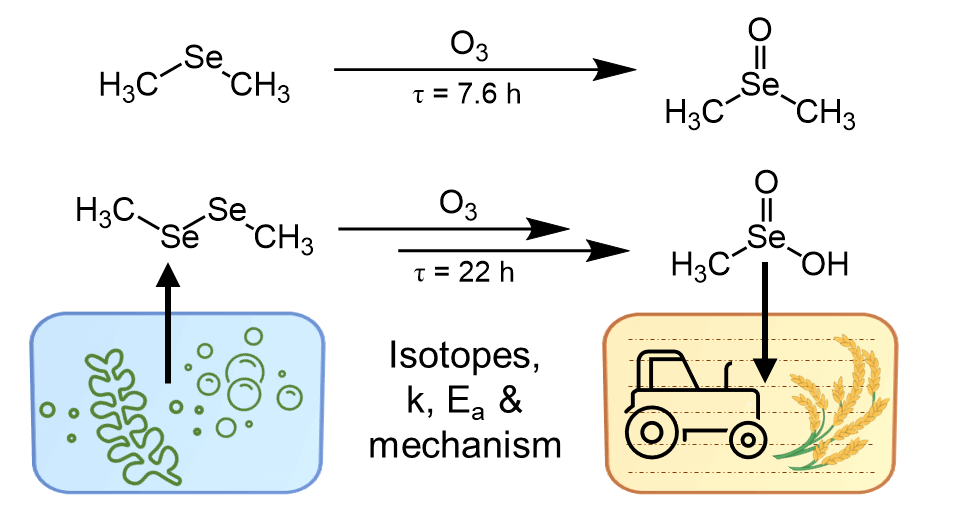
To answer this question, Paul Heine, a graduate student in the research group of Nadine Borduas-Dedekind at the University of British Columbia, conducts laboratory experiments (Figure 2). Heine and Borduas-Dedekind are interested in how Se changes chemically as part of its atmospheric transport through the environment. The researchers use a Vocus proton-transfer-reaction time-of-flight mass spectrometer, a state-of-the-art analytical instrument that detects volatile organic molecules, that are constituents of the atmosphere in relatively small amounts. Interestingly, phytoplankton in marine systems form these volatile organic molecules containing Se, as part of Se’s biogeochemical cycling. Due to their high volatility, these molecules then evaporate into the atmosphere. Here, various physical and chemical transformations driven by light, temperature, humidity, and atmospheric oxidants can occur. In the lab, the researchers use a pillow bag, a reaction vessel for atmospheric chemistry to study these transformations in a controlled environment. Connecting the Vocus to the pillow bag allows the researchers to then monitor the atmospheric oxidation of Se-containing gas-phase molecules in real time.

A recent study, published by Heine and Borduas-Dedekind, identified atmospheric lifetimes and new products of Se containing molecules with respect to their reaction with ozone, a ubiquitous atmospheric oxidant (Figure 3). Moreover, the authors found that the newly identified Se containing reaction products are less volatile, and subsequently condensate onto already existing particles, or directly form new particles. When applying these findings to the atmosphere, Heine and Borduas-Dedekind can then draw conclusions on the atmospheric fate of these Se containing molecules. Oxidation by ozone drives volatile organic compounds containing Se to the particle phase, where they can then be washed out of the atmosphere by rainfall to subsequently be deposited onto soils. The chemical information on reaction times and products of the atmospheric oxidation of volatile Se obtained in this study can be used in computer models estimating the atmospheric flux of Se. With this work, the authors ultimately contribute to improving our predictive capabilities of how Se is distributed globally, and which regions may be at risk of being Se deficient in soils.
Paul Heine is a PhD candidate at the University of British Columbia (UBC) under the supervision of Nadine Borduas-Dedekind (NBD). With a background in organic and inorganic synthesis, Paul has joined the NBD reserach group in 2021 to use the Vocus proton-transfer-reaction time-of-flight mass spectrometer and other analytical tools to study the oxidative fate of volatile organic selenium in the atmosphere. Outside of the lab, Paul enjoys cycling and ski touring. You can find Paul on Twitter (now X) @paul_a_heine, and to learn more about the NBD research group please visit atmoschemgroup.org
Bibliography
Heine, P. A.; Borduas-Dedekind, N. The Ozonolysis of Methylated Selenide Compounds in the Atmosphere: Isotopes, Kinetics, Products, and Mechanisms. Environ. Sci. Technol. 2023. https://doi.org/10.1021/acs.est.3c01586.
atmospheric selenium, Nadine Borduas-Dedekind, ozone, Paul Heine, proton-transfer-reaction-time-of-flight mass spectrometer, selenium
